Fc gamma receptor polymorphisms in antibody therapy: implications for bioassay development to enhance product quality
The Fc Review:
Kicking off a new series where we take a closer look at recent Fc-focused papers. What they found, and why it matters for antibody discovery and development.
How much does FcγR genetic variation influence an antibody’s function?
A recent FDA review examines the often-overlooked role of Fcγ receptor (FcγR) polymorphisms in shaping therapeutic antibody activity, and the implications for the assays used to measure it.
Background:
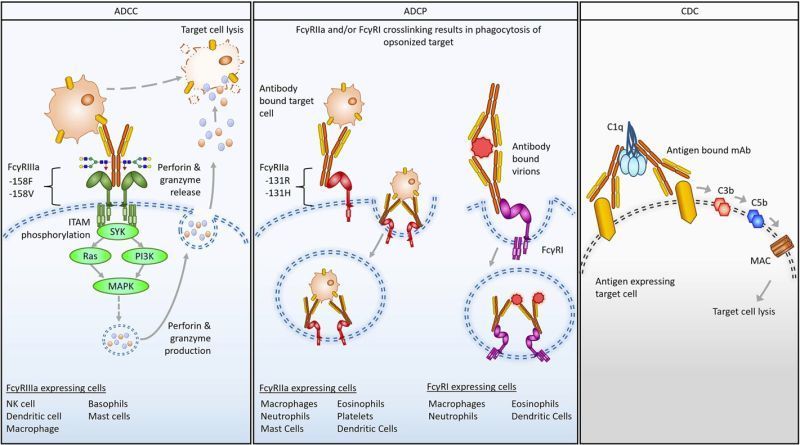
FcγRs are the “effector arm” connection between antibodies and immune cells, driving processes like ADCC and ADCP. Genetic variation in these receptors can alter binding strength, modulate effector function, and impact clinical outcomes. Understanding this interplay is important for therapeutic design, potency assessment, and patient response prediction.
The review highlights:
- Certain FcγR allotypes (e.g., FcγRIIIa-158V/F) can meaningfully shift clinical efficacy and should be considered early in development.
- Primary-cell data provides context for how antibodies interact with real human immune systems, essential for understanding clinical potential.
- Early incorporation of physiologically relevant systems enables detection of functional distinctions that may be missed in oversimplified models.
- Orthogonal approaches like SPR/BLI provide valuable binding context to complement cell-based readouts.
Implications:
The paper underscores the need to balance biological relevance with assay consistency, and to consider receptor diversity early in the development pipeline.
Our perspective:
Evaluating antibodies in systems that reflect human immune responses from the start can reveal functional differences that might otherwise go unnoticed.